PFP (enzyme)
| Diphosphate—fructose-6-phosphate 1-phosphotransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
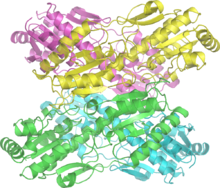 Bacillus stearothermophilus phosphofructokinase.[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.1.90 | ||||||||
| CAS no. | 55326-40-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
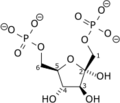
Diphosphate—fructose-6-phosphate 1-phosphotransferase also known as PFP is an enzyme of carbohydrate metabolism in plants and some bacteria. The enzyme (EC 2.7.1.90) catalyses the reversible interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate using inorganic pyrophosphate as the phosphoryl donor:
- diphosphate + D-fructose 6-phosphate phosphate + D-fructose 1,6-bisphosphate
In plants, the PFP is located in the cytosol of the cell and is strongly activated by the signal molecule fructose 2,6-bisphosphate.
PFP is an exclusively cytosolic enzyme that catalyses the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate in the glycolytic direction, and the de-phosphorylation of fructose-1,6-bisphosphate to fructose-6-phosphate in the gluconeogenic reaction. Reeves[2] first isolated PFP from Entamoeba histolytica, a lower eukaryote. The first plant PFP isolated was from the leaves of pineapples by Carnal and Black[3] and it has since been isolated from a variety of plant species and tissues.[4]
Nomenclature
[edit]This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is diphosphate:D-fructose-6-phosphate 1-phosphotransferase. Other names in common use include:
- 6-phosphofructokinase (pyrophosphate),
- inorganic pyrophosphate-dependent phosphofructokinase,
- inorganic pyrophosphate-phosphofructokinase,
- pyrophosphate-dependent phosphofructo-1-kinase, and
- pyrophosphate-fructose 6-phosphate 1-phosphotransferase,
- pyrophosphate-fructose 6-phosphate phosphotransferase
See also
[edit]References
[edit]- ^ PDB: 6PFK; Schirmer T, Evans PR (January 1990). "Structural basis of the allosteric behaviour of phosphofructokinase". Nature. 343 (6254): 140–5. doi:10.1038/343140a0. PMID 2136935.
- ^ Reeves RE, South DJ, Blytt HJ, Warren LG (December 1974). "Pyrophosphate:D-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function of 6-phosphofructokinase". J. Biol. Chem. 249 (24): 7737–41. PMID 4372217.
- ^ Carnal NW, Black CC (January 1979). "Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves". Biochem. Biophys. Res. Commun. 86 (1): 20–6. doi:10.1016/0006-291X(79)90376-0. PMID 219853.
- ^ Stitt M (June 1990). "Fructose-2,6-Bisphosphate as a Regulatory Molecule in Plants". Annual Review of Plant Physiology and Plant Molecular Biology. 41: 153–185. doi:10.1146/annurev.pp.41.060190.001101.
Further reading
[edit]