Talk:Molecular orbital
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||
| |||||||||||||||||||||
The meaning of molecular orbitals
[edit]I strongly disagree with much of the format of the paper, and in particular with the definition given: "In chemistry, a molecular orbital (or MO) is a region in which an electron may be found in a molecule."
I know it's a definition often found in non-quantum chemistry books (organic chemistry, biology, general chemistry...) but to me it's essentially meaningless.
Then the definition continues:"Molecular orbitals are described by wave functions, mathematical solutions to the Schrödinger wave equation for a molecule, [...]" "They can be quantitatively approximated using the Hartree-Fock or Self-Consistent Field method."
Molecular orbitals are NOT solutions to the molecular Schrodinger equation; they arise in the context of independent-particle models/approximation of the exact Schrodinger equation, in particular the Hartree--Fock one. They are not "quantitatively approximated" approximated my the Hartree-Fock method, they are defined by it. —Preceding unsigned comment added by 128.40.5.101 (talk) 19:12, 30 August 2008 (UTC)
- Can a regular, expert contributor respond to these questions? Thanks. — Preceding unsigned comment added by 12.40.94.210 (talk) 20:53, 11 December 2013 (UTC)
- The original comment was made in August 2008. At that time the first sentence (a MO is a region ...) was definitely wrong, but the present version is much better. There were also a few later references to region which I have made somewhat more precise.
- As for the molecular Schrödinger equation, in August 2008 the article DID add the words using an approximation known as the Hartree-Fock or Self-Consistent Field method. This reference to the Hartree-Fock method has since been removed, so I have restored it today. So now the article has been modified to address both criticisms. Dirac66 (talk) 02:49, 16 December 2013 (UTC)
From PNA/Physics
[edit]- Molecular orbital Unclear, poorly wikified. I know a lot more about atomic orbitals than molecular ones, so I'm unwilling to do much here. --Smack 03:58, 14 Oct 2004 (UTC)
- Also see: orbital hybridisation, atomic_orbital, electron_orbital, and sp2 bond. And as Smack says above, electron configuration. Lot's of conceptual overlap. rmbh 01:31, Nov 22, 2004 (UTC)
- I think I have done the job for Molecular orbital. I suggest to merge electron orbital with atomic orbital or simply delete it 17:55, 14 July 2005 (UTC)
The start of the page is (still? again?) really awful. I'll try to improve the beginning and Overview to start - the overview surely should be simple and not introduce lots of concepts only needed if you want heavy detail.... The first picture doesn't help much, being mainly of atomic orbitals. Dump it? Ian 09:18, 30 July 2007 (UTC)
Stub?
[edit]9/7/2004 2105 MST
I'd argue that that this is a stub. It has quite a bit of information, plenty for the casual observer, but by the standard of someone who wants to do anything with it, not enough. If I wanted to put together a FMO diagram for LiH (classic problem) I'm not sure I could after reading this page.
A really nice page. However a couple of things aren't transparent,
Molecular symmetries]? map [stationary states]? to stationary states, so any collection of degenerate? molecular orbitals must transform according to some representation? of the [symmetry group]?. As a result, basis orbitals that transform according to different representations don't mix.
Could you make this a little clearer please, I can't make head nor tail of it.
Also
On the other hand consider a hypothetical molecule of H3, with the atoms labelled H, H', H". Then we would expect three low energy combinations:
1s + 1s" - 1s' Symmetric
1s - 1s" Antisymmetric
1s + 1s' + 1s" Symmetric
Is this correct ? The second combination has onlt 2 basis states and it's not blatenly obvious why the first is symmetric.
Theresa Knott
- I didn't see anything out of order with a brief skim of the article, though I'll have to look at it at length to be sure (it looks like you've found significant typos, at minimum). It reads like it was ripped out of an undergrad chemistry or physics text, though, and could probably be rewritten to be half the length. --Christopher Thomas 07:17, 5 Mar 2005 (UTC)
Is the LIGHT YEARS comment really correct? Got a tad confused there, can someone just see if that is a typo or something??
Ohm
- Yes. It is correct. See Entanglement. Actually I am confused too, even being able to think in functional space. --GS 16:47, 11 May 2005 (UTC)
The discussion on H3 is mostly incorrect. H3 is not C2v symmetry. It is a regular triangle - i.e. D3h. Many people of course discuss linear H3 as the transition structure for H exchange - H + H2 -> H2 + H, but the section here is neither of these things. I suggest someone who has more feel for the overall purpose of this article than I have has a go correcting it, but I might have a go later.Bduke 23:34, 1 November 2005 (UTC)
Actually, H3 does not have a minimum at D3h symmetry. Since there are three electrons, the lowest MO will be doubly-occupied, and at D3h, the remaining electron must be shared over a degenerate MO. This will Jahn-Teller distort away--probably to C2v. User:Ecbrown 20:47, 12 November 2006 (UTC)
Handwaving
[edit]This term handwaving sounds like American baloney to me. It is imprecise. When I was in school "hand waving" implied that mathematics failed, and the lecturer resorted to emphatic hand gestures to convince you he was right. It seems typical of American science that such a poorly understood colloquial term would end up in an encyclopedia. Whoever wrote that ought to get a job at NASA Frizb 16:11, 22 May 2006 (UTC)
- Agree. Done. --Bduke 01:16, 23 May 2006 (UTC)
idea
[edit]The article might be a bit easier to read if the first sentence was split into two or three. Too many ideas in one sentence I think.
- I think most of it wants to be moved further down. If one takes a many determinant wave function one does not have to use molecular orbitals, so this is misleading as the first sentence. Molecular orbitals are used to produce the best single deteminant function. Even that is over-complex to start off. I'll try to think about it, but I'm really on wikibreak and fly to the other side of the world in a few day. --Bduke 12:13, 24 August 2006 (UTC)
- Yes, the intro is too verbose; I will clean it a bit. --Sadi Carnot 17:23, 16 September 2006 (UTC)
Sigma and Pi Bond Order incorrect?
[edit]I think that the Dinitrogen example in section:MO diagrams is incorrect. Isn't the sigma bond order equal to the total number of electrons in sigma orbitals minus the total number of antibonding electrons in sigma orbitals all over 2?
Someone who knows more than me about this please make the correction.130.102.128.60 01:48, 25 July 2007 (UTC)
- Spot on. I've fixed it. --Bduke 03:34, 25 July 2007 (UTC)
History of Orbital Definition
[edit]While presenting at the 1979 Sanibel Symposium, I had the occasion to ask Per-Olov Löwdin for his definition of an orbital. Without hesitation, he told me that it was first used by Robert S. Mulliken "in 1925 as the English translation of Schroedinger's use of the German word, 'Eigenfunktion'." Mulliken had been working in Germany in 1925 with many of the founders of Quantum Mechanics and particularly in 1927 with Friedrich Hund on the beginnings of molecular orbital theory. Mulliken presented his work at that time in Physical Review. Löwdin told me to look there for the first use of a hydrogen "orbital" in English. He also agreed that a suitable definition of an orbital would be "a mathematical function that describes the wave-like behavior of an electron". I will edit the definition to reflect this usage of the term as a mathematical function, but also connect it to the more general description of an orbital as a "region of space" that can be calculated from the function. The advent of graphing calculators has enabled even high school students to grasp the definition of an orbital as a mathematical function. I invite educators to have their students graph the "simplified' 1s orbital, y = exp(-abs(Z.x)), where Z=1,2,3(Atomic Number). This simple exercise can display the effects of electronegativity and the fact that atoms and isoelectronic ions "decrease" their size in going to the right in a row in the periodic chart. The bonding and antibonding sigma MO can also be displayed by y = exp(-abs(Zx+1) + or - exp(-abs(Zx-1) . After graphing functions, students can grasp the idea of "combining" functions (atomic orbitals) to get other functions (molecular orbitals), and what is meant by the + and - signs or the red/blue, white/gray colors of the orbitals. As for some measure of proof, please see
- Mulliken's 1966 Nobel acceptance speech[1]
Laburke (talk) 22:26, 21 November 2008 (UTC)
- I fully support your edits. They are an improvement. The source above does need adding however. I was not at Sanibel in 1979 but I was in 1982 and 1990, and I recall some others. --Bduke (Discussion) 22:38, 21 November 2008 (UTC)
- This is weird! The cited Nobel speech clearly states:
- Among other changes, atomic electron orbits were replaced by atomic orbitals, although the name orbital was given only later, in 1932.
- How does that fit with your 1925 dating? —Preceding unsigned comment added by 134.176.250.16 (talk) 23:07, 15 February 2010 (UTC)
There is not doubt that Mulliken first used the term "orbital", but the date is a problem. I doubt it is 1925 given that the publication of the Schrodinger equation was 1926. I have therefore removed it. This needs a source. What Per-Olov Löwdin told Laburke will not do, although I too remember Per-Olov, at a Sanibel meeting, crediting Mulliken with the use of the term, but I do not recall him mentioning a date. -Bduke (Discussion) 23:34, 15 February 2010 (UTC)
- Thanks for clarifying this. Schroedinger's equation dated to 1926 was one reason for my doubts. On the other hand, I don't think this allows saying he can't have used the "Eigenfunktion"-term in 1925, when Mulliken was in Germany...
- I did some searching and found [[2]], which itself is hardly citable but has references to possible "first used here"-articles. The mentioned 1932 article states:
- From here on, one-electron orbital wave functions will be referred to for brevity as orbitals
- ...which seems to be the introduction of "orbitals", though just as abbreviation for "orbital wave functions". I don't see where the "orbital" in there comes from?! And maybe related: Who did the shift from "orbit" to "orbital"? From "the path the electron takes around the core" to "a region where it might be"? (Does one have to distinguish between the term and the idea it describes? Both don't necessarily attribute to the same person!)
- After all I'm still confused!
- Or is my remaining problem just an orthographic one? Is orbital in "orbital wave functions" a noun or an adjective? If the latter all is good! —Preceding unsigned comment added by 134.176.250.25 (talk) 20:07, 16 February 2010 (UTC)
Please excuse the typographical error. I meant to write "in the 1920s..." not "in 1925" Laburke (talk) 03:24, 28 April 2010 (UTC)
- This is for your expert consideration. I'm not one, but I was taught by some. I found in conversation, the late Jeremy Burdett at Chicago combined deep understanding and interesting historical comments. These could not have been first hand. He might be considered 2nd generation. His books might have insights into the history that would help here. (He once explained conversationally how he chose the style of orbital drawings in Molecular Shapes. The explanation highlighted personal observations: that one proponent in the early days was earnest they should be shaded, another that they should be colored, etc. Humorous and humanizing.) In areas where his work and my field overlap, they are outstanding teaching resources.) His books are listed here: http://www.independent.co.uk/news/obituaries/obituary--professor-jeremy-burdett-1252237.html or here http://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Dstripbooks&field-keywords=Jeremy+Burdett+orbital. A person who trained with him, and who also might be a good resource for you, is John F. Mitchell, at Argonne National Lab, here http://www.msd.anl.gov/mitchell. Now, he works more with materials. But he might offer good comments on subtle issues, or suggestion someone else to. Thank you all for your hard work here. — Preceding unsigned comment added by 12.40.94.210 (talk) 21:19, 11 December 2013 (UTC)
Major edits underway
[edit]A group of chemistry undergraduates including myself have started major edits and additions as a class project in an effort to have the article upgrade to an 'A' rating or better. It will take several weeks or months for us to finish. -- 11:47, 4 February 2011 (UTC)
- The latest edit contains several errors, both in scientific content and in wikimedia markup. For the latter you have changed the categories so that they are merely listed and do not actually put the article in the categories. I have fixed that. You need to get up to speed with the technical issues of writing articles. For the scientific content, I need to spend more time on but the statement that the Hartree-Fock orbitals converge fast in CI to full CI is simply not true. The virtual HF MOs are not good MOs for CI. Someone said, correctly, that they are the junk left over when you have found the optimized occupied orbitals. I also think you confuse the difference between AOs and basis functions. They are not the same. --Bduke (Discussion) 00:59, 21 May 2011 (UTC)
- Also appears to includes major copyright violation: images scanned from a commercial textbook. What school is this? The class professor or WP:AMBASSADOR may want to review appropriate content with the students so they don't get blocked even before the project progresses further. DMacks (talk) 07:03, 21 May 2011 (UTC)
File:S orbital interactions.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:S orbital interactions.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:08, 21 May 2011 (UTC) |
File:HF MO diagram.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:HF MO diagram.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:08, 21 May 2011 (UTC) |
File:H2 MO diagram.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:H2 MO diagram.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:08, 21 May 2011 (UTC) |
File:AO alignment.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:AO alignment.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:09, 21 May 2011 (UTC) |
File:Diatomic MO bonding data table.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:Diatomic MO bonding data table.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:26, 21 May 2011 (UTC) |
File:He2 Li2 MO diagram.jpg Nominated for speedy Deletion
[edit]An image used in this article, File:He2 Li2 MO diagram.jpg, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:26, 21 May 2011 (UTC) |
File:P orbital interactions.png Nominated for speedy Deletion
[edit]An image used in this article, File:P orbital interactions.png, has been nominated for speedy deletion at Wikimedia Commons for the following reason: Copyright violations
| |
| Speedy deletions at commons tend to take longer than they do on Wikipedia, so there is no rush to respond. If you feel the deletion can be contested then please do so (commons:COM:SPEEDY has further information). Otherwise consider finding a replacement image before deletion occurs.
A further notification will be placed when/if the image is deleted. This notification is provided by a Bot, currently under trial --CommonsNotificationBot (talk) 07:27, 21 May 2011 (UTC) |
Missing figure
[edit]In the section Molecular orbital there's a reference to a figure 6, while in the whole of the lemma only three pictures apear. As on this talk page there are some notifications of pictures to be removed from the commons, I suspect the problem is (partly) in this removal. I don't feel confident enough on the subject to correct the numbering, so please .....:-) T.vanschaik (talk) 07:10, 21 November 2012 (UTC)
- Yup, they were all removed as copyvio. I rewrote the content to describe what would have been seen, and removed the "figure #" notations from the existing images precisely because it's so fragile towards future edits. DMacks (talk) 12:43, 21 November 2012 (UTC)
- Please, please, Bduke, DMacks, other experts, verify student editing mistakes mentioned above have been removed. If not, can you please edit? Please bring it back to your earlier expert status. The errors in this type of article are even for a practicing chemist that is not an MO theory specialist to catch on a quick read. We need to know the article is reliable, so we can refer students here to learn. Thank you. — Preceding unsigned comment added by 12.40.94.210 (talk) 20:50, 11 December 2013 (UTC)
Comment on citations
[edit]I have no reason to doubt the quality of this article. But it's troubling that some parts have no or few refs. Need to add refs, so sources are clear and to fulfill WP:VERIFY. It gives readers a way to go deeper in their study. It makes the content traceable, so questions that arise can be resolved starting at the ref. (Maybe the ref is not representative. Maybe it's a poor ref. Maybe it even has mistakes?) Until there are refs throughout, I can't send students here to learn. — Preceding unsigned comment added by 12.40.94.210 (talk) 20:42, 11 December 2013 (UTC)
Acetylene figure good choice. (But)
[edit]A good choice because acetylene is a little familiar. And good because it is a simple, linear molecule. But a student or other interested reader will have no clue what they are looking at. Maybe: Start with saying what the two-tone grey is peeking out (connect to H-C-C-H). Then explain the blue and red. Then make clear why two columns, and why multiple rows. If a ref can be given, good too. Thanks. — Preceding unsigned comment added by 12.40.94.210 (talk) 21:26, 11 December 2013 (UTC)
- Done, except that I don't know the original reference. Dirac66 (talk) 22:30, 18 December 2013 (UTC)
Possible 2nd fig for Homonuclear Diatomics sub-section?
[edit]The cycling movie/cartoon has a good, clear legend. (You could move the last sentence up.) The acetylene fig leg could use this kind of expansion.
You might consider adding a dihydrogen MO diagram. Having the MO diagr helps connect the good, active cartoon, to more widely seen figs. Even if deciding not to, the ordinate (energy) should be labeled, so it's clear that sigma is lower and sigma-star is higher in energy. Here are possible figs, one including g and u:
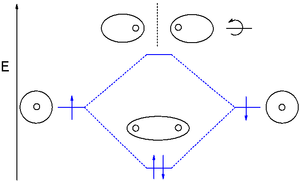

This would address confusion comparing double peak appearance in cartoon, and oval-of-revolution / spheres-touching-at-a-point ways of representing the MOs. And, Walsh mention anywhere? Thanks for work. — Preceding unsigned comment added by 12.40.94.210 (talk) 21:49, 11 December 2013 (UTC)
"Dumbing down"
[edit]In the header, "on an elementary level" redirects to "dumbing down". I assume this is a troll that has gone unnoticed so far. Can someone remove it?
--2A02:8070:E284:B100:6DC0:5429:40B4:DBC0 (talk) 21:41, 14 November 2017 (UTC)
- Removed. Thank you. Dirac66 (talk) 23:02, 14 November 2017 (UTC)
- C-Class level-4 vital articles
- Wikipedia level-4 vital articles in Physical sciences
- C-Class vital articles in Physical sciences
- C-Class physics articles
- Top-importance physics articles
- C-Class physics articles of Top-importance
- C-Class Chemistry articles
- Top-importance Chemistry articles
- WikiProject Chemistry articles


