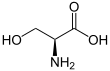
Serine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Serine
| |||
| Other names
2-Amino-3-hydroxypropanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.250 | ||
| EC Number |
| ||
| |||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[2] | |||
| C3H7NO3 | |||
| Molar mass | 105.093 g·mol−1 | ||
| Appearance | white crystals or powder | ||
| Density | 1.603 g/cm3 (22 °C) | ||
| Melting point | 246 °C (475 °F; 519 K) decomposes | ||
| soluble | |||
| Acidity (pKa) | 2.21 (carboxyl), 9.15 (amino)[1] | ||
| Supplementary data page | |||
| Serine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Serine (symbol Ser or S)[3][4] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO−
form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.
Occurrence
[edit]
This compound is one of the proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer.[5] Its name is derived from the Latin for silk, sericum. Serine's structure was established in 1902.[6][7]
Biosynthesis
[edit]The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate (an intermediate from glycolysis) to 3-phosphohydroxypyruvate and NADH by phosphoglycerate dehydrogenase (EC 1.1.1.95). Reductive amination (transamination) of this ketone by phosphoserine transaminase (EC 2.6.1.52) yields 3-phosphoserine (O-phosphoserine) which is hydrolyzed to serine by phosphoserine phosphatase (EC 3.1.3.3).[8][9]
In bacteria such as E. coli these enzymes are encoded by the genes serA (EC 1.1.1.95), serC (EC 2.6.1.52), and serB (EC 3.1.3.3).[10]

Serine hydroxymethyltransferase (SMHT) also catalyzes the biosynthesis of glycine (retro-aldol cleavage) from serine, transferring the resulting formalddehyde synthon to 5,6,7,8-tetrahydrofolate. However, that reaction is reversible, and will convert excess glycine to serine.[11] SHMT is a pyridoxal phosphate (PLP) dependent enzyme.[8]
Synthesis and reactions
[edit]Industrially, L-serine is produced from glycine and methanol catalyzed by hydroxymethyltransferase.[12]
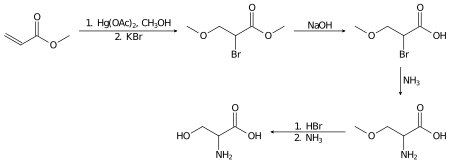
Racemic serine can be prepared in the laboratory from methyl acrylate in several steps:[13]
Hydrogenation of serine gives the diol serinol:
- HOCH2CH(NH2)CO2H + 2 H2 → HOCH2CH(NH2)CH2OH + 2 H2O
Biological function
[edit]Metabolic
[edit]
Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, as well as tryptophan in bacteria. It is also the precursor to numerous other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis.[citation needed]
Signaling
[edit]D-Serine, synthesized in neurons by serine racemase from L-serine (its enantiomer), serves as a neuromodulator by coactivating NMDA receptors, making them able to open if they then also bind glutamate. D-serine is a potent agonist at the glycine site (NR1) of canonical diheteromeric NMDA receptors. For the receptor to open, glutamate and either glycine or D-serine must bind to it; in addition a pore blocker must not be bound (e.g. Mg2+ or Zn2+).[14] Some research has shown that D-serine is a more potent agonist at the NMDAR glycine site than glycine itself.[15][16] However, D-serine has been shown to work as an antagonist/inverse co-agonist of t-NMDA receptors through the glycine binding site on the GluN3 subunit.[17][18]
Ligands
[edit]D-serine was thought to exist only in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signaling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site.[19] Apart from central nervous system, D-serine plays a signaling role in peripheral tissues and organs such as cartilage,[20] kidney,[21] and corpus cavernosum.[22]
Gustatory sensation
[edit]Pure D-serine is an off-white crystalline powder with a very faint musty aroma. D-Serine is sweet with an additional minor sour taste at medium and high concentrations.[23]
Clinical significance
[edit]Serine deficiency disorders are rare defects in the biosynthesis of the amino acid L-serine. At present three disorders have been reported:
- 3-phosphoglycerate dehydrogenase deficiency
- 3-phosphoserine phosphatase deficiency
- Phosphoserine aminotransferase deficiency
These enzyme defects lead to severe neurological symptoms such as congenital microcephaly and severe psychomotor retardation and in addition, in patients with 3-phosphoglycerate dehydrogenase deficiency to intractable seizures. These symptoms respond to a variable degree to treatment with L-serine, sometimes combined with glycine.[24][25] Response to treatment is variable and the long-term and functional outcome is unknown. To provide a basis for improving the understanding of the epidemiology, genotype/phenotype correlation and outcome of these diseases their impact on the quality of life of patients, as well as for evaluating diagnostic and therapeutic strategies a patient registry was established by the noncommercial International Working Group on Neurotransmitter Related Disorders (iNTD).[26]
Besides disruption of serine biosynthesis, its transport may also become disrupted. One example is spastic tetraplegia, thin corpus callosum, and progressive microcephaly, a disease caused by mutations that affect the function of the neutral amino acid transporter A.
Research for therapeutic use
[edit]The classification of L-serine as a non-essential amino acid has come to be considered as conditional, since vertebrates such as humans cannot always synthesize optimal quantities over entire lifespans.[27] Safety of L-serine has been demonstrated in an FDA-approved human phase I clinical trial with Amyotrophic Lateral Sclerosis, ALS, patients (ClinicalTrials.gov identifier: NCT01835782),[28][29] but treatment of ALS symptoms has yet to be shown. A 2011 meta-analysis found adjunctive sarcosine to have a medium effect size for negative and total symptoms of schizophrenia.[30] There also is evidence that L‐serine could acquire a therapeutic role in diabetes.[31]
D-Serine is being studied in rodents as a potential treatment for schizophrenia.[32] D-Serine also has been described as a potential biomarker for early Alzheimer's disease (AD) diagnosis, due to a relatively high concentration of it in the cerebrospinal fluid of probable AD patients.[33] D-serine, which is made in the brain, has been shown to work as an antagonist/inverse co-agonist of t-NMDA receptors mitigating neuron loss in an animal model of temporal lobe epilepsy.[34]
D-Serine has been theorized as a potential treatment for sensorineural hearing disorders such as hearing loss and tinnitus.[35]
See also
[edit]- Isoserine
- Homoserine (isothreonine)
- Serine octamer cluster
References
[edit]- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Weast RC, ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-512. ISBN 0-8493-0462-8.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)". Pure and Applied Chemistry. 56 (5): 595–624. 1984. doi:10.1351/pac198456050595..
- ^ Cramer E (1865). "Ueber die Bestandtheile der Seide" [On the constituents of silk]. Journal für praktische Chemie (in German). 96: 76–98. Serine is named on p. 93: "Ich werde den in Frage stehenden Körper unter dem Namen Serin beschreiben." (I will describe the body [i.e., substance] in question by the name "serine".)
- ^ Fischer E, Leuchs H (1902). "Synthese des Serins, der l-Glucosaminsäure und anderer Oxyaminosäuren" [Synthesis of serine, of l-glucosaminic acid, and other oxyamino acids]. Berichte der Deutschen Chemischen Gesellschaft (in German). 35 (3): 3787–3805. doi:10.1002/cber.190203503213.
- ^ "Serine". The Columbia Encyclopedia 6th ed. encyclopedia.com. Retrieved 22 October 2012.
- ^ a b Stryer L (1988). Biochemistry (3rd ed.). New York: W.H. Freeman. p. 580. ISBN 978-0-7167-1843-7.
- ^ KEGG EC 3.1.3.3 etc.
- ^ Uniprot: serB
- ^ Lehninger AL, Nelson DL, Cox MM (2000). Principles of Biochemistry (3rd ed.). New York: W. H. Freeman. ISBN 1-57259-153-6.
- ^ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker (2006). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Carter HE, West HD (1940). "dl-Serine". Org. Synth. 20: 81. doi:10.15227/orgsyn.020.0081.
- ^ Liu Y, Hill RH, Arhem P, von Euler G (2001). "NMDA and glycine regulate the affinity of the Mg2+-block site in NR1-1a/NR2A NMDA receptor channels expressed in Xenopus oocytes". Life Sciences. 68 (16): 1817–1826. doi:10.1016/S0024-3205(01)00975-4. PMID 11292060.
- ^ MacKay MA, Kravtsenyuk M, Thomas R, Mitchell ND, Dursun SM, Baker GB (6 February 2019). "D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression?". Frontiers in Psychiatry. 10: 25. doi:10.3389/fpsyt.2019.00025. ISSN 1664-0640. PMC 6372501. PMID 30787885.
D-Serine is more potent than glycine as a coagonist at the NMDA receptor, has a regional distribution in the brain that is similar to that of NMDA receptors and appears to be more closely associated with synaptic NMDA receptors than glycine (which is more closely associated with non-synaptic NMDA receptors).
- ^ Wolosker H, Balu DT (9 June 2020). "D-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders". Translational Psychiatry. 10 (1): 184. doi:10.1038/s41398-020-00870-x. ISSN 2158-3188. PMC 7283225. PMID 32518273.
D-Serine is functionally a more potent activator of synaptic NMDARs than glycine, and mounting evidence suggests that it serves as the major NMDAR co-agonist in limbic brain regions implicated in neuropsychiatric disorders.
- ^ Pilli J, Kumar SS (2012-10-11). "Triheteromeric N-methyl-D-aspartate receptors differentiate synaptic inputs onto pyramidal neurons in somatosensory cortex: involvement of the GluN3A subunit". Neuroscience. 222: 75–88. doi:10.1016/j.neuroscience.2012.07.020. ISSN 1873-7544. PMID 22814002. S2CID 23158971.
- ^ Beesley S, Kumar SS (2023-11-01). "The t-N-methyl-d-aspartate receptor: Making the case for d-Serine to be considered its inverse co-agonist". Neuropharmacology. 238: 109654. doi:10.1016/j.neuropharm.2023.109654. ISSN 1873-7064. PMID 37437688.
- ^ Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, Rogawski MA, Snyder SH (Apr 2000). "D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor". Proceedings of the National Academy of Sciences of the United States of America. 97 (9): 4926–4931. Bibcode:2000PNAS...97.4926M. doi:10.1073/pnas.97.9.4926. PMC 18334. PMID 10781100.
- ^ Takarada T, Hinoi E, Takahata Y, Yoneda Y (May 2008). "Serine racemase suppresses chondrogenic differentiation in cartilage in a Sox9-dependent manner". Journal of Cellular Physiology. 215 (2): 320–328. doi:10.1002/jcp.21310. PMID 17929246. S2CID 45669104.
- ^ Ma MC, Huang HS, Chen YS, Lee SH (Nov 2008). "Mechanosensitive N-methyl-D-aspartate receptors contribute to sensory activation in the rat renal pelvis". Hypertension. 52 (5): 938–944. doi:10.1161/HYPERTENSIONAHA.108.114116. PMID 18809793.
- ^ Ghasemi M, Rezania F, Lewin J, Moore KP, Mani AR (Jun 2010). "D-Serine modulates neurogenic relaxation in rat corpus cavernosum". Biochemical Pharmacology. 79 (12): 1791–1796. doi:10.1016/j.bcp.2010.02.007. PMID 20170643.
- ^ Kawai M, Sekine-Hayakawa Y, Okiyama A, Ninomiya Y (Dec 2012). "Gustatory sensation of L- and D-amino acids in humans". Amino Acids. 43 (6): 2349–2358. doi:10.1007/s00726-012-1315-x. PMID 22588481. S2CID 17671611.
- ^ de Koning TJ (April 2006). "Treatment with amino acids in serine deficiency disorders". Journal of Inherited Metabolic Disease. 29 (2): 347–351. doi:10.1007/s10545-006-0269-0. PMID 16763900. S2CID 25013468.
- ^ Tabatabaie L, Klomp LW, Berger R, de Koning TJ (March 2010). "L-Serine synthesis in the central nervous system: a review on serine deficiency disorders". Mol Genet Metab. 99 (3): 256–262. doi:10.1016/j.ymgme.2009.10.012. PMID 19963421.
- ^ "Patient registry".
- ^ Metcalf JS, Dunlop RA, Powell JT, Banack SA, Cox PA (2017). "L-Serine: a Naturally-Occurring Amino Acid with Therapeutic Potential". Neurotoxicity Research. 33 (1): 213–221. doi:10.1007/s12640-017-9814-x. ISSN 1029-8428. PMID 28929385. S2CID 20271849.
- ^ Dunlop RA, Cox PA, Banack SA, Rodgers KJ (2013). "The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation". PLOS ONE. 8 (9): e75376. Bibcode:2013PLoSO...875376D. doi:10.1371/journal.pone.0075376. PMC 3783393. PMID 24086518.
- ^ Levine TD, Miller RG, Bradley WG, Moore DH, Saperstein DS, Flynn LE, Katz JS, Forshew DA, Metcalf JS, Banack SA, Cox PA (2017-01-02). "Phase I clinical trial of safety of L-serine for ALS patients". Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 18 (1–2): 107–111. doi:10.1080/21678421.2016.1221971. ISSN 2167-8421. PMID 27589995. S2CID 4584977.
- ^ Singh SP, Singh V (Oct 2011). "Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia". CNS Drugs. 25 (10): 859–885. doi:10.2165/11586650-000000000-00000. PMID 21936588. S2CID 207299820.
- ^ Holm LJ, Buschard K (2019). "L-serine: a neglected amino acid with a potential therapeutic role in diabetes". APMIS. 127 (10): 655–659. doi:10.1111/apm.12987. ISSN 0903-4641. PMC 6851881. PMID 31344283.
- ^ Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT (Jun 2013). "Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction". Proceedings of the National Academy of Sciences of the United States of America. 110 (26): E2400–E2409. Bibcode:2013PNAS..110E2400B. doi:10.1073/pnas.1304308110. PMC 3696825. PMID 23729812.
- ^ Madeira C, Lourenco MV, Vargas-Lopes C, Suemoto CK, Brandão CO, Reis T, Leite RE, Laks J, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Ferreira ST, Panizzutti R (May 5, 2015). "D-Serine levels in Alzheimer's disease: implications for novel biomarker development". Translational Psychiatry. 5 (5): e561. doi:10.1038/tp.2015.52. PMC 4471283. PMID 25942042.
- ^ Beesley S, Sullenberger T, Crotty K, Ailani R, D'Orio C, Evans K, Ogunkunle EO, Roper MG, Kumar SS (2020-10-02). "D-serine mitigates cell loss associated with temporal lobe epilepsy". Nature Communications. 11 (1): 4966. Bibcode:2020NatCo..11.4966B. doi:10.1038/s41467-020-18757-2. ISSN 2041-1723. PMC 7532172. PMID 33009404.
- ^ Wang J, Serratrice N, Lee CJ, François F, Sweedler JV, Puel JL, Mothet JP, Ruel J (17 December 2021). "Physiopathological Relevance of D-Serine in the Mammalian Cochlea". Frontiers in Cellular Neuroscience. 15. Frontiers Media SA: 733004. doi:10.3389/fncel.2021.733004. ISSN 1662-5102. PMC 8718999. PMID 34975405.