Chlorodifluoromethane
| |||
 Liquefied chlorodifluoromethane boiling when exposed to STP
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloro(difluoro)methane | |||
| Other names
Chlorodifluoromethane
Difluoromonochloromethane Monochlorodifluoromethane HCFC-22 R-22 Genetron 22 Freon 22 Arcton 4 Arcton 22 UN 1018 Difluorochloromethane Fluorocarbon-22 Refrigerant 22 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.793 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1018 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
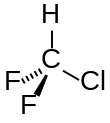
| CHClF2 | |||
| Molar mass | 86.47 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Sweetish[1] | ||
| Density | 3.66 kg/m3 at 15 °C, gas | ||
| Melting point | −175.42 °C (−283.76 °F; 97.73 K) | ||
| Boiling point | −40.7 °C (−41.3 °F; 232.5 K) | ||
| 0.7799 vol/vol at 25 °C; 3.628 g/L | |||
| log P | 1.08 | ||
| Vapor pressure | 908 kPa at 20 °C | ||
Henry's law
constant (kH) |
0.033 mol⋅kg−1⋅bar−1 | ||
| −38.6·10−6 cm3/mol | |||
| Structure | |||
| Tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Dangerous for the environment (N), Central nervous system depressant, Carc. Cat. 3 | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H420 | |||
| P202, P262, P271, P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | nonflammable[1] | ||
| 632 °C (1,170 °F; 905 K) | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
None[1] | ||
REL (Recommended)
|
TWA 1000 ppm (3500 mg/m3) ST 1250 ppm (4375 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or CHClF
2. It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.

Production and current applications
[edit]Worldwide production of R-22 in 2008 was about 800 Gg per year, up from about 450 Gg per year in 1998, with most production in developing countries.[2] R-22 use is being phased out in developing countries, where it is largely used for air conditioning applications.
R-22 is prepared from chloroform:
- HCCl3 + 2 HF → HCF2Cl + 2 HCl
An important application of R-22 is as a precursor to tetrafluoroethylene. This conversion involves pyrolysis to give difluorocarbene, which dimerizes:[3]
- 2 CHClF2 → C2F4 + 2 HCl
The compound also yields difluorocarbene upon treatment with strong base and is used in the laboratory as a source of this reactive intermediate.
The pyrolysis of R-22 in the presence of chlorofluoromethane gives hexafluorobenzene.
Environmental effects
[edit]R-22 is often used as an alternative to the highly ozone-depleting CFC-11 and CFC-12, because of its relatively low ozone depletion potential of 0.055,[4] among the lowest for chlorine-containing haloalkanes. However, even this lower ozone depletion potential is no longer considered acceptable.
As an additional environmental concern, R-22 is a powerful greenhouse gas with a GWP equal to 1810 (which indicates 1810 times as powerful as carbon dioxide). Hydrofluorocarbons (HFCs) are often substituted for R-22 because of their lower ozone depletion potential, but these refrigerants often have a higher GWP. R-410A, for example, is often substituted, but has a GWP of 2088. Another substitute is R-404A with a GWP of 3900. Other substitute refrigerants are available with low GWP. Ammonia (R-717), with a GWP of <1, remains a popular substitute on fishing vessels and large industrial applications. Ammonia's toxicity in high concentrations limit its application in small-scale refrigeration applications.
Propane (R-290) is another example, and has a GWP of 3. Propane was the de facto refrigerant in systems smaller than industrial scale before the introduction of CFCs. The reputation of propane refrigerators as a fire hazard kept delivered ice and the ice box the overwhelming consumer choice despite its inconvenience and higher cost until safe CFC systems overcame the negative perceptions of refrigerators. Illegal to use as a refrigerant in the US for decades, propane is now permitted for use in limited mass suitable for small refrigerators. It is not lawful to use in air conditioners or larger refrigerators because of its flammability and potential for explosion.
-
HCFC-22 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
-
Growth of R-22 (CFC-22) abundance in Earth's atmosphere since year 1992.[5]
Phaseout in the European Union
[edit]
Since 1 January 2010, it has been illegal to use newly manufactured HCFCs to service refrigeration and air-conditioning equipment; only reclaimed and recycled HCFCs may be used. In practice this means that the gas has to be removed from the equipment before servicing and replaced afterwards, rather than refilling with new gas.
Since 1 January 2015, it has been illegal to use any HCFCs to service refrigeration and air-conditioning equipment; broken equipment that used HCFC refrigerants must be replaced with equipment that does not use them.[6]
Phaseout in the United States
[edit]R-22 was mostly phased out in new equipment in the United States by regulatory action by the EPA under the Significant New Alternatives Program (SNAP) by rules 20 and 21 of the program,[7] due to its high global warming potential. The EPA program was consistent with the Montreal Accords, but international agreements must be ratified by the US Senate to have legal effect. A 2017 decision of the US Court of Appeals for the District of Columbia Circuit[8] held that the US EPA lacked authority to regulate the use of R-22 under SNAP. In essence the court ruled the EPA's statutory authority[9] was for ozone reduction, not global warming. The EPA subsequently issued guidance to the effect that the EPA would no longer regulate R-22. A 2018 ruling[10] by the same court held that the EPA failed to conform with required procedure when it issued its guidance pursuant to the 2017 ruling, voiding the guidance, but not the prior ruling that required it. The refrigeration and air conditioning industry had already discontinued production of new R-22 equipment. The practical effect of these rulings is to reduce the cost of imported R-22 to maintain aging equipment, extending its service life, while preventing the use of R-22 in new equipment.
R-22, retrofit using substitute refrigerants
[edit]The energy efficiency and system capacity of systems designed for R-22 is slightly greater using R-22 than the available substitutes.[11]
R-407A is for use in low- and medium-temp refrigeration. Uses a polyolester (POE) oil.
R-407C is for use in air conditioning. Uses a minimum of 20 percent POE oil.
R-407F and R-407H are for use in medium- and low-temperature refrigeration applications (supermarkets, cold storage, and process refrigeration); direct expansion system design only. They use a POE oil.
R-421A is for use in "air conditioning split systems, heat pumps, supermarket pak systems, dairy chillers, reach-in storage, bakery applications, refrigerated transport, self-contained display cabinets, and walk-in coolers". Uses mineral oil (MO), Alkylbenzene (AB), and POE.
R-422B is for use in low-, medium-, and high-temperature applications. It is not recommended for use in flooded applications.
R-422C is for use in medium- and low-temperature applications. The TXV power element will need to be changed to a 404A/507A element and critical seals (elastomers) may need to be replaced.
R-422D is for use in low-temp applications, and is mineral oil compatible.
R-424A is for use in air conditioning as well as medium-temp refrigeration temperature ranges of 20 to 50˚F. It works with MO, alkylbenzenes (AB), and POE oils.
R-427A is for use in air conditioning and refrigeration applications. It does not require all the mineral oil to be removed. It works with MO, AB, and POE oils.
R-434A is for use in water cooled and process chillers for air conditioning and medium- and low-temperature applications. It works with MO, AB, and POE oils.
R-438A (MO-99) is for use in low-, medium-, and high-temperature applications. It is compatible with all lubricants. [12]
R-458A is for use in air conditioning and refrigeration applications, without capacity or efficiency loss. Works with MO, AB, and POE oils.[13]
R-32 or HFC-32 (difluoromethane) is for use in air conditioning and refrigeration applications. It has zero ozone depletion potential (ODP) [2] and a global warming potential (GWP) index 675 times that of carbon dioxide.
Physical properties
[edit]| Property | Value |
|---|---|
| Density (ρ) at −69 °C (liquid) | 1.49 g⋅cm−3 |
| Density (ρ) at −41 °C (liquid) | 1.413 g⋅cm−3 |
| Density (ρ) at −41 °C (gas) | 4.706 kg⋅m−3 |
| Density (ρ) at 15 °C (gas) | 3.66 kg⋅m−3 |
| Specific gravity at 21 °C (gas) | 3.08 (air is 1) |
| Specific volume (ν) at 21 °C (gas) | 0.275 m3⋅kg−1 |
| Density (ρ) at 15 °C (gas) | 3.66 kg⋅m−3 |
| Triple point temperature (Tt) | −157.39 °C (115.76 K) |
| Critical temperature (Tc) | 96.2 °C (369.3 K) |
| Critical pressure (pc) | 4.936 MPa (49.36 bar) |
| Vapor pressure at 21.1 °C (pc) | 0.9384 MPa (9.384 bar)[14] |
| Critical density (ρc) | 6.1 mol⋅l−1 |
| Latent heat of vaporization (lv) at boiling point (−40.7 °C) | 233.95 kJ⋅kg−1 |
| Heat capacity at constant pressure (Cp) at 30 °C (86 °F) | 0.057 kJ.mol−1⋅K−1 |
| Heat capacity at constant volume (Cv) at 30 °C (86 °F) | 0.048 kJ⋅mol−1⋅K−1 |
| Heat capacity ratio (γ) at 30 °C (86 °F) | 1.178253 |
| Compressibility factor (Z) at 15 °C | 0.9831 |
| Acentric factor (ω) | 0.22082 |
| Molecular dipole moment | 1.458 D |
| Viscosity (η) at 0 °C | 12.56 μPa⋅s (0.1256 cP) |
| Ozone depletion potential (ODP) | 0.055 (CCl3F is 1) |
| Global warming potential (GWP) | 1810 (CO2 is 1) |
It has two allotropes: crystalline II below 59 K and crystalline I above 59 K and below 115.73 K.

| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg K) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number | Bulk modulus (K^-1) |
|---|---|---|---|---|---|---|---|---|
| 230 | 1416 | 1.087 | 3.56E-04 | 2.51E-07 | 0.1145 | 7.44E-08 | 3.4 | 0.00205 |
| 240 | 1386.6 | 1.1 | 3.15E-04 | 2.27E-07 | 0.1098 | 7.20E-08 | 3.2 | 0.00216 |
| 250 | 1356.3 | 1.117 | 2.80E-04 | 2.06E-07 | 0.1052 | 6.95E-08 | 3 | 0.00229 |
| 260 | 1324.9 | 1.137 | 2.50E-04 | 1.88E-07 | 0.1007 | 6.68E-08 | 2.8 | 0.00245 |
| 270 | 1292.1 | 1.161 | 2.24E-04 | 1.73E-07 | 0.0962 | 6.41E-08 | 2.7 | 0.00263 |
| 280 | 1257.9 | 1.189 | 2.01E-04 | 1.59E-07 | 0.0917 | 6.13E-08 | 2.6 | 0.00286 |
| 290 | 1221.7 | 1.223 | 1.80E-04 | 1.47E-07 | 0.0872 | 5.83E-08 | 2.5 | 0.00315 |
| 300 | 1183.4 | 1.265 | 1.61E-04 | 1.36E-07 | 0.0826 | 5.52E-08 | 2.5 | 0.00351 |
| 310 | 1142.2 | 1.319 | 1.44E-04 | 1.26E-07 | 0.0781 | 5.18E-08 | 2.4 | 0.004 |
| 320 | 1097.4 | 1.391 | 1.28E-04 | 1.17E-07 | 0.0734 | 4.81E-08 | 2.4 | 0.00469 |
| 330 | 1047.5 | 1.495 | 1.13E-04 | 1.08E-07 | 0.0686 | 4.38E-08 | 2.5 | 0.00575 |
| 340 | 990.1 | 1.665 | 9.80E-05 | 9.89E-08 | 0.0636 | 3.86E-08 | 2.6 | 0.00756 |
| 350 | 920.1 | 1.997 | 8.31E-05 | 9.04E-08 | 0.0583 | 3.17E-08 | 2.8 | 0.01135 |
| 360 | 823.4 | 3.001 | 6.68E-05 | 8.11E-08 | 0.0531 | 2.15E-08 | 3.8 | 0.02388 |
Price history and availability
[edit]
EPA's analysis indicated the amount of existing inventory was between 22,700t and 45,400t.[17][18][when?]
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015–2019 | 2020 |
|---|---|---|---|---|---|---|---|
| R-22 Virgin (t) | 49,900 | 45,400 | 25,100 | 25,600 | 20,200 | TBD | 0 |
| R-22 Recoupment (t) | -- | -- | -- | 2,950 | 2,950 | -- | -- |
| R-22 Total (t) | 49,900 | 45,400 | 25,100 | 28,600 | 23,100 | -- | -- |
In 2012 the EPA reduced the amount of R-22 by 45%, causing the price to rise by more than 300%. For 2013, the EPA has reduced the amount of R-22 by 29%.[19]
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0124". National Institute for Occupational Safety and Health (NIOSH).
- ^ Rosenthal, Elisabeth; Lehren, Andrew W. (20 June 2012). "Relief in Every Window, but Global Worry Too". The New York Times. Archived from the original on 21 June 2012. Retrieved 21 June 2012.
- ^ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Sart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3527306732.
- ^ The Montreal Protocol on Substances that Deplete the Ozone Layer. UNEP, 2000. ISBN 92-807-1888-6
- ^ "HCFC-22 (Chlorodifluoromethane)". NOAA Earth System Research Laboratories/Global Monitoring Division. Retrieved 12 February 2021.
- ^ "Guidance for Stationary Refrigeration & Air-Conditioning" (PDF). Department for Environment, Food and Rural Affairs. Archived (PDF) from the original on 10 March 2016. Retrieved 8 September 2015.
- ^ "SNAP Regulations". 4 November 2014. Archived from the original on 10 October 2015.
- ^ "Mexichem Fluor, Inc. v. EPA". Archived from the original on 17 August 2017.
- ^ "Ozone Protection under Title VI of the Clean Air Act". 14 July 2015. Archived from the original on 25 January 2016.
- ^ "Natural Resources Defense Council v. EPA". Archived from the original on 10 December 2020.
- ^ "THEORETICAL EVALUATION OF R22 AND R502 ALTERNATIVES" (PDF). Archived (PDF) from the original on 5 April 2015.
- ^ Retrofit Refrigerants Archived 24 June 2013 at archive.today
- ^ "Protection of Stratospheric Ozone: Determination 33 for Significant New Alternatives Policy Program". 21 July 2017.
- ^ "Frogen R-22 – Frogen UK: Refrigerant and Cooling Specialists". frogen.co.uk. Archived from the original on 25 January 2017. Retrieved 23 April 2018.
- ^ Holman, Jack P. (2002). Heat Transfer (9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606. ISBN 978-0-07-240655-9.
- ^ Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 978-0-471-45728-2.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ "Protection of Stratospheric Ozone: Adjustments to the Allowance System for Controlling HCFC Production, Import, and Export". federalregister.gov. 3 April 2013. Archived from the original on 4 March 2016. Retrieved 23 April 2018.
- ^ "Protection of Stratospheric Ozone: Adjustments to the Allowance System for Controlling HCFC Production, Import, and Export". federalregister.gov. 3 April 2013. Archived from the original on 4 March 2016. Retrieved 23 April 2018.
- ^ Specialty Cooling and Heating (Blog) January 22, 2013 Archived 6 October 2013 at the Wayback Machine
External links
[edit]- MSDS from DuPont
- International Chemical Safety Card 0049
- Data at Integrated Risk Information System: IRIS 0657
- CDC – NIOSH Pocket Guide to Chemical Hazards – Chlorodifluoromethane
- Phase change data at webbook.nist.gov
- IR absorption spectra Archived 28 November 2007 at the Wayback Machine
- IARC summaries and evaluations: Vol. 41 (1986), Suppl. 7 (1987), Vol. 71 (1999)




![Growth of R-22 (CFC-22) abundance in Earth's atmosphere since year 1992.[5]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/cf/HCFC22_concentration.jpg/591px-HCFC22_concentration.jpg)